Electron Spinning in an ATOM
We have discussed in our previous article (Semiconductor Physics:- Electron Configuration In Atom, about the atoms (and analogy) and the basic idea of electron movement. If you remember, we have finished our article just introducing the spining of electrons in 3D dimensions and about the quantum numbers
n - principal quantum number – This is same number as we know orbital no.
l - azimuthal quantum number – This is same as we know s,p,d,f
m - magnetic quantum number.
"Among the three quantum numbers, there is another quantization condition called spin (s) that electrons should fulfill. Every electron has its angular momentum or spin and can be expressed as..."
Before going through the periodic table, there is the Pauli exclusion principle which stated that no two electrons have the same quantum numbers (n, l, m, and s). In other words, two electrons may have the same quantum number n, l, and m states but the spin states must be +1/2 and -1/2.
The configuration of electrons in different energy states is summarized in Table 1.
Table 1 The allowed energy states and the occupation of maximum number of electrons according to quantum rules.
As an example, for the 3rd electronic orbit (n=3), l should be 0, 1, and 2 followed by equation 14. Similarly, m should be -l to +l including zero for each l value. Electrons can occupy each m value with a spin +/-1/2.
The different notations for different l values are used to express the electronic configuration
l = 0,1,2,3,4,5 .......
l = s, p, d, f, g, h, .....
The electron state can be represented as
I think now you are able to understand whole electron configuration which you have studied in your college days. Let’s talk about few more examples, so that you can be familiar with Semiconductor and Physics very well.
For example, Si (Atomic No=14) and Ge (Atomic No= 32), the electronic configuration is
Si 1s22s22p63s23p2
Figure 5 Electronic configuration of Silicon
Ge 1s22s22p63s23p64s23d104p2
Figure 6 Electronic configuration of germanium
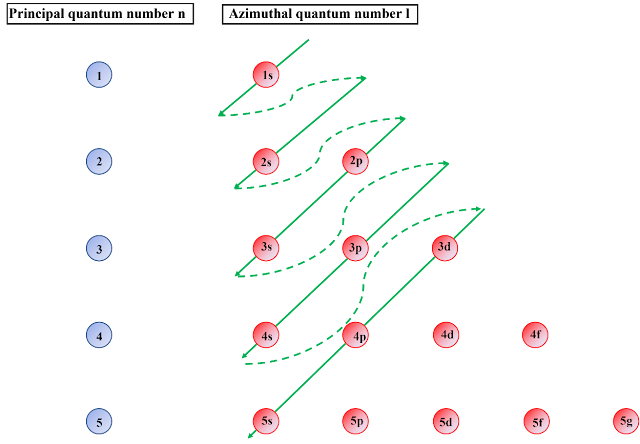
The electronic arrangements are shown in figure1
Figure 7 Electron’s configuration rule
In the next article, we will discuss the different type of bonding between the ATOMS and the formation of energy bands which will help us to understand the Semiconductor Physics more deeply and later semiconductor Devices like PN Diode, MOSFETs and all.
By -
First Author - Dr. Bhaskar Patnayak
Second Author (Editor) - Mr. Puneet Mittal








No comments:
Post a Comment